This is the first of two articles on the outlook for and potential timing of treatments and vaccines for COVID-19. The goal is to provide practical assessments of the health care industry’s efforts for business leaders and public health officials who need to plan for the future of their companies and communities. In this article we examine the landscape of possible treatments. It is based on broader research conducted by the BCG scientist network, which comprises more than 500 individuals around the world with advanced degrees (MDs and PhDs) and relevant private-sector experience.
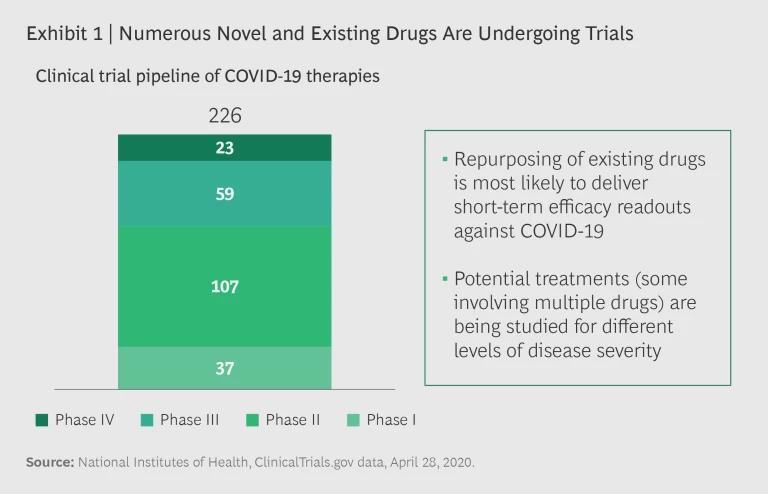
Potential treatments for COVID-19 are evolving with unprecedented speed, and data and results from several clinical trials are expected in the coming months. More than 200 active clinical trials are underway, including multiple trials for the same drugs. (See Exhibit 1.) Several trials seek to establish the efficacy of existing therapies, including more than 80 Phase III and IV trials. There are positive signs of potential benefits across multiple drug classes and candidates.
While the key to resolving the COVID-19 crisis remains the development of a vaccine, that is likely a year or more away. In the meantime, effective treatments not only can save lives, they can reduce the strain on the health care system—in terms of both capacity and finances—and help restore public and business confidence. Crises can breed misinformation and exaggeration, and a plethora of stories about the potential efficacy of multiple drugs have proliferated, frequently without much supporting evidence. The truth is usually more complicated and nuanced than what is covered in the headlines.
Therapeutic Strategies
Potential treatments so far fall into two broad categories:
- Antivirals aimed at limiting the spread of the virus inside the bodies of infected patients
- Immune-based therapies that prevent viral entry and limit the damage that the body inflicts upon itself while fighting off the virus
Both types of treatment can help reduce the morbidity and mortality of the disease until a safe and effective vaccine is brought to market at scale.
Antivirals
A virus invades a body’s normal cells (such as respiratory cells in the lungs) and hijacks the cell division machinery to create replicas of itself. To overcome the body’s immune defenses, a virus must enter a sufficient number of host cells and continuously replicate; therefore, a key antiviral drug strategy is to block viral replication.
Effective treatments can not only save lives, they can relieve the strain on the health care system.
Four existing medications aimed at blocking viral replication are under investigation as potential COVID-19 treatments: chloroquine/hydroxychloroquine, remdesivir, litonavir/lopinavir, and interferon beta. Some clinical data is already being made public about these drugs, and much more will become available in the coming months. It is likely that each drug will have differing efficacies for individual patient segments (depending on such factors as disease severity). Here are the clinical facts on the two highest-profile therapies.
Chloroquine/Hydroxychloroquine. These are antimalarial drugs that gained attention based on anecdotal evidence early in the pandemic. They generated significant enthusiasm because they are oral agents whose production can be quickly scaled up to meet global demand. Current evidence on their effectiveness as treatment agents is inconclusive, however. Several studies are ongoing, most notably the National Institutes of Health ORCHID study, from which preliminary results are expected this autumn.
Chloroquine, and hydroxychloroquine in particular, are also being considered for prophylactic use, that is, as preventive agents. The EPICOS study in Spain is assessing the hydroxychloroquine’s ability to prevent COVID-19 in health care workers and will likely provide data late in the second quarter of 2020. A larger study of 40,000 people is also underway but will likely not have data available until 2021. A potential limiting factor is the long-term side effects of chloroquine and hydroxychloroquine, which include abnormal heart rhythm and ocular damage.
While we await data, the US Food and Drug Administration has issued a warning advising against the use of chloroquine and hydroxychloroquine outside of clinical trials.
Remdesivir. This intravenous drug from Gilead Sciences, which has received a lot of public attention recently, was developed in 2015 as a possible treatment for Ebola. Remdesivir was identified by Gilead as a potential treatment for COVID-19 following a screening effort across the company’s portfolio of antiviral drugs. Gilead quickly established clinical trials in January at multiple sites around the world to assess its potential.
On April 29, the FDA moved to authorize emergency use of remdesivir.
On April 29, investigators announced interim results from a global, placebo-controlled trial, sponsored by the National Institute of Allergy and Infectious Diseases, which demonstrated that remdesivir shortened the time to clinical recovery from a median of 15 days in the control group to 11 days in the group receiving the drug. By reducing lengthy inpatient care, this could help hospitals better manage capacity. Also on April 29, the FDA moved to authorize emergency use of remdesivir.
On the same day, the results from one of two randomized, open-label, multicenter Phase III clinical trials (the SIMPLE studies)—which Gilead had initiated in countries with a high prevalence of COVID-19 infection—were released and showed equivalent outcomes for a five-day versus a ten-day treatment course. This is important, as demand will likely be high, and a five-day treatment will allow twice as many patients to receive the drug. Gilead has stated publicly that it expects to donate about 1.5 million doses of remdesivir for ongoing clinical trials, expanded access, and compassionate use. This is equivalent to approximately 280,000 five-day treatments.
Gilead has also stated that it is increasing production of remdesivir to meet the expected demand, with an additional 360,000 to 500,000 treatment courses expected by October. Remdesivir is not currently produced commercially, so a commercial-scale supply chain needs to be rapidly created to deliver it in time and in the anticipated required volumes. These developments are significant early steps in building a portfolio of therapeutics to treat COVID-19.
Immune System-Based Therapies
Given the importance of the immune response to viral infections, researchers have focused on two facets of immunology for COVID treatment: neutralizing antibodies to prevent viral entry and immune modulators to treat hyperreactivity to infection. Of the two, neutralizing antibodies will likely have applications in a broader patient population. Multiple companies (such as Regeneron, Eli Lilly, and Vir) have efforts underway to develop antibody cocktails, which will likely enter trials in the summer. Emerging data on immune modulators suggests that the most promising application may be in the most severely ill patients, as these agents have shown benefits in those receiving mechanical ventilation.
Neutralizing Antibodies. Patients who recover from COVID-19 typically generate antibodies against the virus. Convalescent plasma and hyperimmune globulin are two neutralizing antibody therapies that have entered clinical trials in the US, following favorable results from observational studies in China. (See Exhibit 2.) Convalescent plasma treatment involves purifying the blood serum from recovered patients and administering it to high-risk individuals. Convalescent plasma can be used either as a prophylaxis to prevent initial infection or as a treatment to limit the spread of the infection among cells. While the approach has proven effective for high-risk groups—particularly frontline health care workers—in previous viral outbreaks (such as H1N1 flu and Ebola), it is challenging to manufacture the plasma at scale. According to FDA guidelines, one donor can provide three treatment units of convalescent plasma, which can treat six patients.
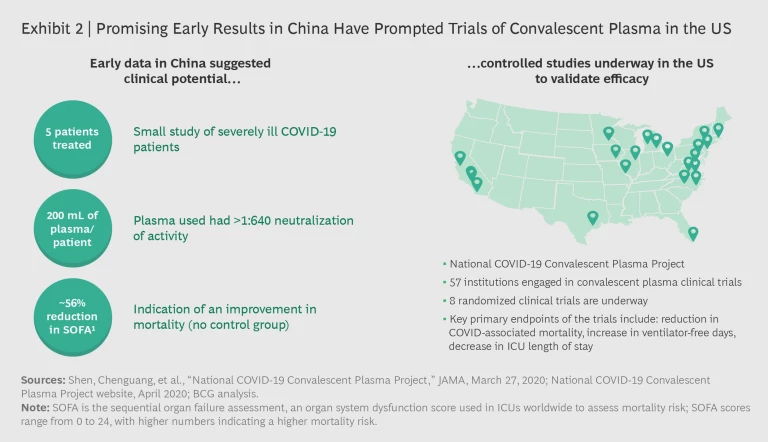
The National COVID-19 Convalescent Plasma Project, initiated by researchers at a number of US institutions, has developed a network of sites at 57 academic institutions to biobank plasma. Results from trials in two New York City hospitals are expected soon. If the data is positive, there will likely be a need to increase public awareness in order to find more donors. We will also need more testing to identify patients with sufficient neutralizing antibody titers for donation. Given supply constraints on novel therapeutics such as remdesivir, such a network will be key to ensuring access for specific patient groups to the therapies that will benefit them most.
Hyperimmune globulin is a concentrated form of convalescent plasma that requires additional purification to produce. Based on data from China that showed positive results, a group of industry partners (including CSL Behring, Takeda, Biotest, BPL, LFB, and Octapharma) have established the CoVIg-19 Plasma Alliance with the goal of producing hyperimmune globulin on a mass scale. The alliance has already established 500 collection centers in the US. Phase III trials exploring treatment with the plasma are scheduled to begin in June 2020, with results available by September 2020.
Convalescent plasma can be used either as a prophylaxis to prevent initial infection or as a treatment to limit the spread of the infection among cells.
Immune Modulators. COVID-19 patients can suffer significant damage to the lungs caused by a hyperactive immune-system response to the infection. Researchers are investigating drugs that can modulate this response. Tocilizumab and sarilumab are two antibody drugs that target interleukin-6 (IL-6), an immune protein whose levels are raised in COVID-19 patients. Results from two early, small studies in China suggest that the drugs may improve survival in severely ill patients. Roche and Sanofi recently launched two large, multinational trials evaluating the effectiveness of the drugs in this patient population. Interim results have validated the earlier studies and final results are expected by mid-May or in June.
Stay ahead with BCG insights on the health care industry
The Clinical Development Timeline
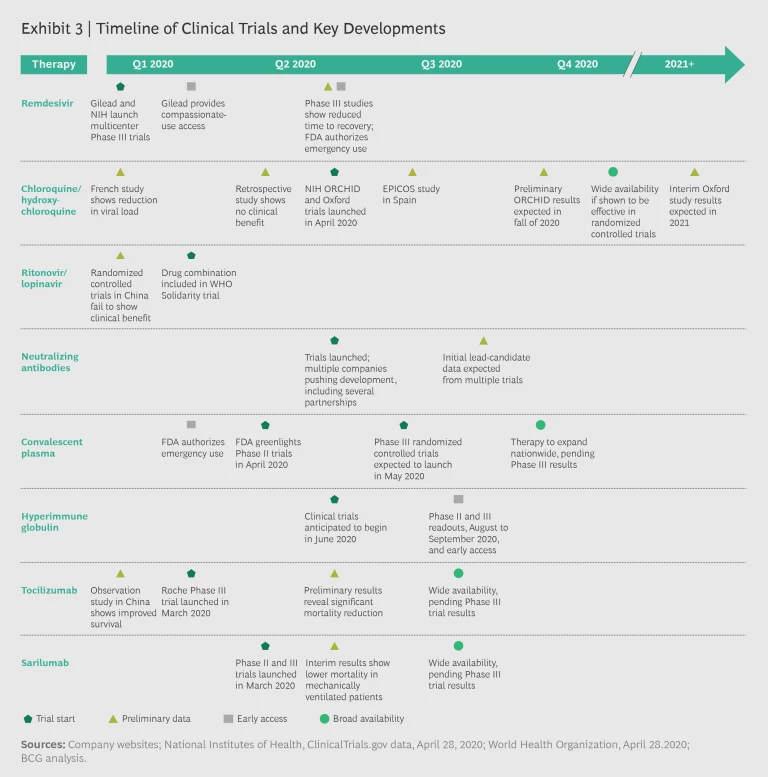
The majority of clinical trials will have preliminary or final results by the third quarter of 2020. Several therapies under investigation may gain approval by the fourth quarter. If clinical data comes back positive, convalescent plasma, hyperimmune globulin, remdesivir, tocilizumab, and sarilumab could conceivably be available for clinical use by year’s end. Exhibit 3 shows the likely timing of major trial results.
To achieve broad access, pharmaceutical companies will need to closely collaborate with regulators around the world and coordinate clinical trials on a global scale. Such coordination will be critical to several success factors, including:
- Accelerating trial completion through pooled data from multiple sites
- Achieving a larger enrollment of patients in multiple locations
- Enabling rapid data monitoring and analysis, as well as the dissemination of new knowledge on effective therapies
Standardizing clinical testing and coordinating the plethora of clinical trials will accelerate the development of safe and effective therapies, in particular by avoiding numerous duplicative and underpowered studies. This will require coordination across countries and strong guidance from organizations such as the World Health Organization. Megatrials such as WHO’s Global Solidarity Project, which involves more than 100 countries, should result in the enrollment of thousands of patients and the monitoring and rapid analysis of clinical data on a global scale. Coordination of trials will also reduce competition for the same scarce resources, thereby alleviating the burden on already overwhelmed health systems.
Three pivotal dates are upcoming that could change the trajectory of COVID-19 and directly affect business planning: remdesivir’s final Phase III trial results in May 2020 for full FDA approval, tociluzumab and sarilumab’s Phase III results anticipated in June 2020, and the NIH’s ORCHID trial results in the fourth quarter. Each of these therapies may fundamentally alter the treatment landscape and bend the mortality curve of the disease. It bears noting that these drugs will likely have existing scale or a strong starting point to meet anticipated demand. But of course, actual demand will depend on the specific patient populations most likely to benefit from each drug.
Three pivotal dates are upcoming that could change the trajectory of COVID-19 and directly affect business planning.
There is also a large effort underway to develop a second wave of therapies more directly targeted at COVID-19. As the first wave of drugs gains approval, the second will likely face a higher bar for safety and efficacy. Authorization of redmesivir for emergency use based on interim results released the same day highlights the speed at which companies, regulators, and governments are moving to find a treatment. We will likely begin to see additional actions over the coming months although so far the data suggests that a portfolio of drugs for specific patient populations is more likely than a single cure.
In the short term, each new therapy will probably be specific to a subset of patients. But as more therapies become available, treatment coverage will broaden. Growing improvement in patient outcomes (in terms of both reduced hospital stays and reduced mortality) will increase confidence in a return to a more normal way of life. There may not be a single cure, but there will be a growing suite of tools to help protect ourselves until we achieve a safe and effective vaccine at scale.